ISSN: 1449-2288International Journal of Biological Sciences
Int J Biol Sci 2024; 20(5):1815-1832. doi:10.7150/ijbs.85562 This issue Cite
Research Paper
Ubiquitin specific protease 38 aggravates pathological cardiac remodeling by stabilizing phospho-TBK1
1. Department of Cardiology, Renmin Hospital of Wuhan University, Wuhan, China.
2. Hubei Key Laboratory of Cardiology, Wuhan, China.
3. Cardiovascular Research Institute of Wuhan University, Wuhan, China.
4. Department of Respiratory Medicine, Hubei RongJun Hospital, Wuhan, China.
†Zheng Xiao, Chang Dai and Tingting Yu contributed equally.
Abstract
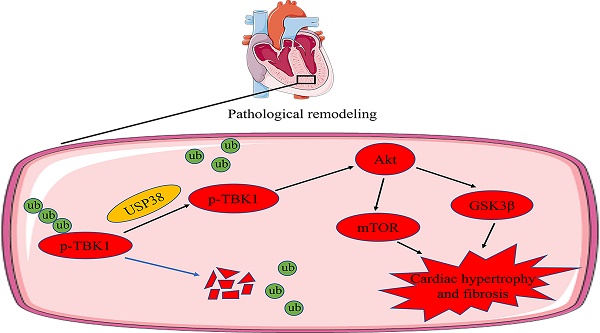
Chronic pressure overload can cause pathological cardiac remodeling and eventually heart failure. The ubiquitin specific protease (USP) family proteins play a prominent role in regulating substrate protein degradation and cardiac structural and functional homeostasis. Although USP38 is expressed in the heart, uncertainty exists regarding the function of USP38 in pathological cardiac remodeling. We constructed and generated cardiac specific USP38 knockout mice and cardiac specific USP38 overexpression mice to assess the role of USP38 in pathological cardiac remodeling. Furthermore, we used co-immunoprecipitation (Co-IP) assays and western blot analysis to identify the molecular interaction events. Here, we reported that the expression of USP38 is significantly elevated under a hypertrophic condition in vivo and in vitro. USP38 deletion significantly mitigates cardiomyocyte enlargement in vitro and hypertrophic effect induced by pressure overload, while overexpression of USP38 markedly aggravates cardiac hypertrophy and remodeling. Mechanistically, USP38 interacts with TANK-binding kinase 1 (TBK1) and removes K48-linked polyubiquitination of TBK1, stabilizing p-TBK1 and promoting the activation of its downstream mediators. Overexpression of TBK1 in the heart of cardiac specific USP38 knockout mice partially counteracts the benefit of USP38 deletion on pathological cardiac remodeling. The TBK1 inhibitor Amlexanox significantly alleviates pressure overload induced-cardiac hypertrophy and myocardial fibrosis in mice with USP38 overexpression. Our results demonstrate that USP38 serves as a positive regulator of pathological cardiac remodeling and suggest that targeting the USP38-TBK1 axis is a promising treatment strategy for hypertrophic heart failure.
Keywords: Pathological cardiac remodeling, Heart failure, Ubiquitin specific protease 38, TANK-binding kinase 1
